The E-Commerce Opportunity in China's OTC Pharmaceuticals Market
A new pilot program in Beijing indicates loosening restrictions on the sale of imported OTC pharmaceutical products in China
by Ker Zheng
On December 30th, 2019, the China National Medical Products Administration approved a pilot program for the bonded importing of OTC pharmaceutical products in Beijing. Foreign merchants will be able to store products in Beijing's Tianzhu bonded warehouse area, from which they can fulfill customer orders.
Such OTC products include cold medicines, skin treatment creams, digestive medicines such as laxatives, and certain vitamins and supplements.
While some merchants such as Chemist Warehouse sell on Tmall Global's cross-border e-commerce platform, sales have been hampered by regulations and concerns over the proper handling of such sensitive imported products. Products can only be shipped one-by-one from Australia, rather than from bonded warehouses in China.
Currently, sellers of OTC pharmaceutical products on Tmall Global must place a 300,000 RMB deposit down to cover potential risks.
We take a deeper look at the pilot program and where it fits into the context of China's massive OTC pharmaceuticals market.
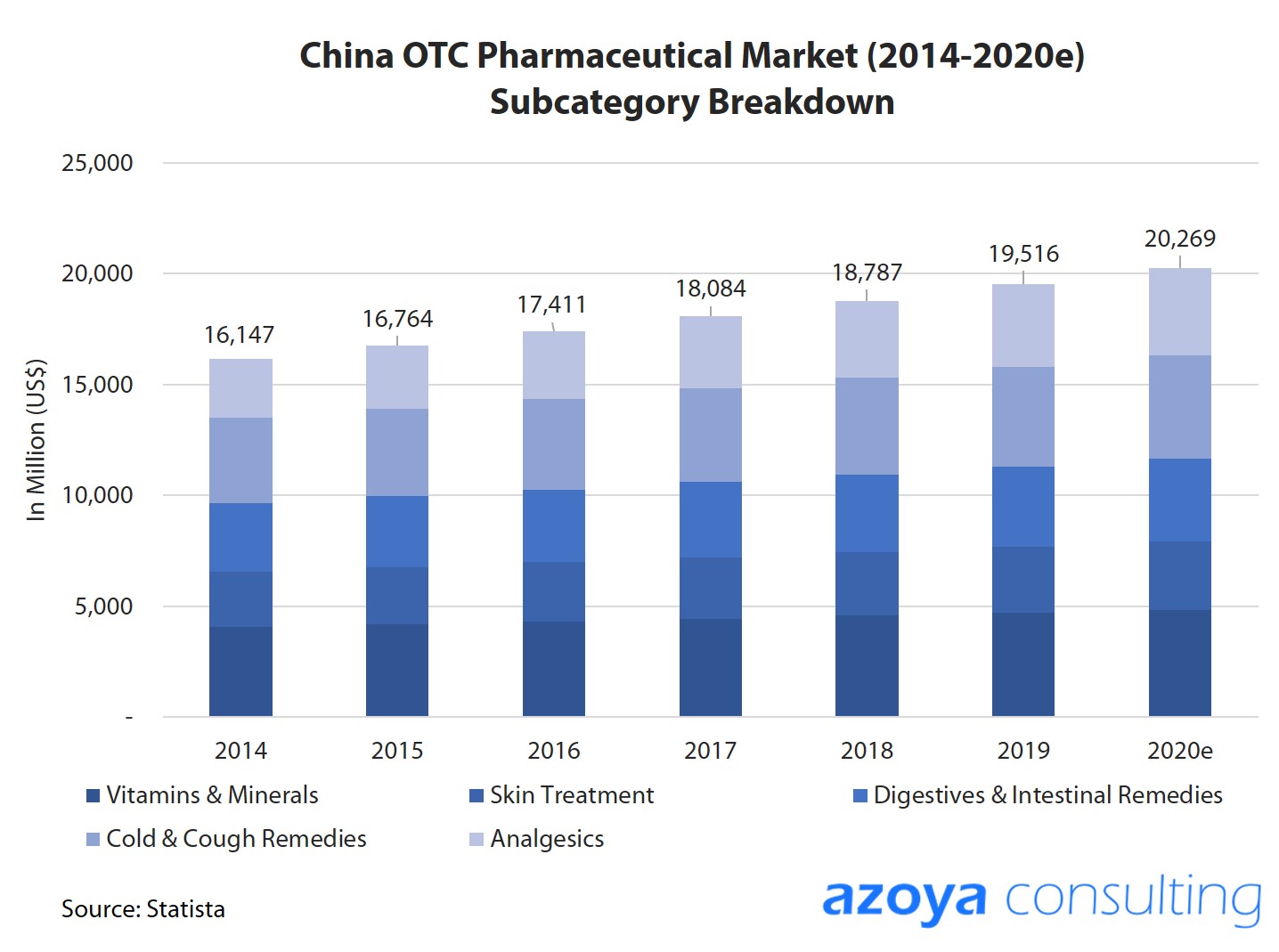
China's OTC Pharmaceuticals Market is A Massive Opportunity
China's $19.5 billion OTC pharmaceutical market is the world's second-largest behind the US, and is expected to reach US$22.7 billion by 2023.
Growth is primarily driven by China's large population, rising consumption levels, and growing awareness of health issues.
For example, Chinese millennials working longer hours in tier 1 cities are increasingly facing issues such as insomnia, fatigue, and weight gain. Instead of going to crowded public hospitals, they are conducting their own research to diagnose and treat minor illnesses, which bodes well for this industry.
Other customers such as China's elderly are purchasing imported products such as laxatives, nicotine patches, etc.
Chemist Warehouse, an online pharmacy in Australia, sells OTC products such as nicotine patches, sore throat lozenges, and eye drop solution on Tmall Global.

A banner on Chemist Warehouse's Tmall Global flagship store
Qualifications for the Pilot Program
Sellers of OTC pharmaceutical products and medical devices are allowed to apply for the program. Here are the requirements:
- The applicant enterprise shall be an enterprise registered in the administrative area of Beijing and have a qualified corporate legal person representing the company. There should also be a third-party e-commerce platform that enables the sale of such products.
- The imported products will be cleared through Beijing customs.
- The importing company should have warehousing capabilities that both 1. meet requirements for the storage of cross-border pharmaceutical imports and 2. be based in Beijing's Tianzhu bonded warehouse area.
- Importing companies should sell through third-party e-commerce platforms that meet the requirements for the pilot program. This includes quality control systems and returns processing of cross-border pharmaceutical products, as well as risk prevention and control systems. Companies should provide pre-sales and after-sales services for customers.
- Products should be pre-registered with the National Medical Products Administration, and the product subcategories should be on the official list issued by the thirteen relevant ministries and commissions.
Remaining Challenges
1. Foreign product certifications by organizations such as America's Food & Drug Administration are not recognized in China. All drugs and medical devices must be approved by the National Medical Products Administration for sale.
2. OTC products are subject to more storage, shipping, and customs clearance requirements, which raises the costs of selling them in China. Shipments to customers may be held back by customs in some cases.
3. Marketing and advertising OTC pharmaceutical products are heavily regulated in China. Many social media platforms limit content/ads on products in this category, and many influencers refuse to help market OTC products as well.
Key Takeaways
1. China's OTC pharmaceutical market is massive and growing rapidly, driven by rising consumption levels and increasing awareness of health issues.
2. A new pilot program in Beijing allows the bonded warehouse importing and selling of foreign OTC pharmaceutical products through cross-border e-commerce
3. Key obstacles remain, such as registration processes, marketing restrictions, etc. However, the government seems keen on further loosening regulations to meet popular demand from Chinese citizens.